Singderm® Mono-phasic Dermal Filler
Singderm® Mono-phasic Dermal Filler is a testament to refined aesthetics and your journey to enhancing your natural beauty. Our dermal filler products are crafted with precision and undergo rigorous testing to ensure they provide safe, effective, and stunning results.
Product Description
Singderm® Mono-phasic Dermal Filler is a testament to refined aesthetics and your journey to enhancing your natural beauty. Our dermal filler products are crafted with precision and undergo rigorous testing to ensure they provide safe, effective, and stunning results. Whether you aim to restore volume, smooth wrinkles, or sculpt facial contours, Singderm® Mono-phasic Dermal Filler offers a range of solutions tailored to your unique desires.
Tags: Facial Volume Restoration, Facial Fillers for Sale, Fillers for Your Face, Dermal Fillers Safe, Cheap Dermal Fillers, Filler Areas, Face Contouring Before and After
Facial Fillers for Sale
As people get older, the hyaluronic acid in the face will be gone, and the volume of the face will lose gradually, this is why wrinkles and folds will appear. However, supplement of hyaluronic acid by injecting hyaluronic acid dermal filler will cause pains. This is why dermal filler with lidocaine is important, lidocaine helps to numb the skin and make the injection more comfortable, it can help reduce pain and discomfort during the injection process.
Singderm® Mono-phasic Dermal Filler is a sterile, biodegradable, nonpyrogenic, viscoelastic, clear, colorless, homogenized gel implant. Mono-phasic Dermal Filler consists of modified hyaluronic acid (HA) produced by bacteria, formulated to a concentration of 24 mg/ml and 0.3% lidocaine in a physiologic buffer. Indicated for injection into the mid to deep dermis or subcutaneous for correction of mid or deep depressions of the skin, as well as for correcting age-related volume deficit.
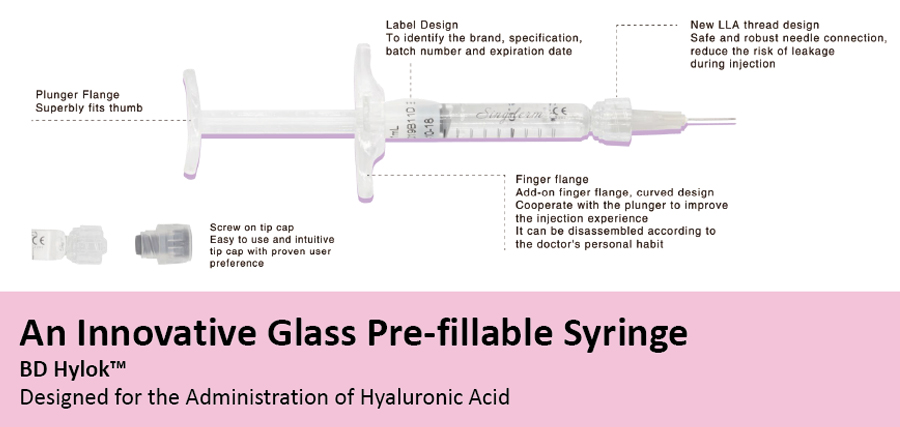
Singderm® is presented in a graduated, pre-filled, disposable syringe. Each box contains one syringe, an instruction leaflet and a set of labels in order to ensure traceability. The contents of the Singderm® syringe are sterilized by moist heat. If presense, the needles are sterilized by radiation or ethylene oxide.
Intended Use
Singderm® Mono-phasic Dermal Filler is indicated for facial tissue augmentation by injection into areas in which restoration is required, including reconstructive treatment of volume loss as well as for facial morphological asymmetry.
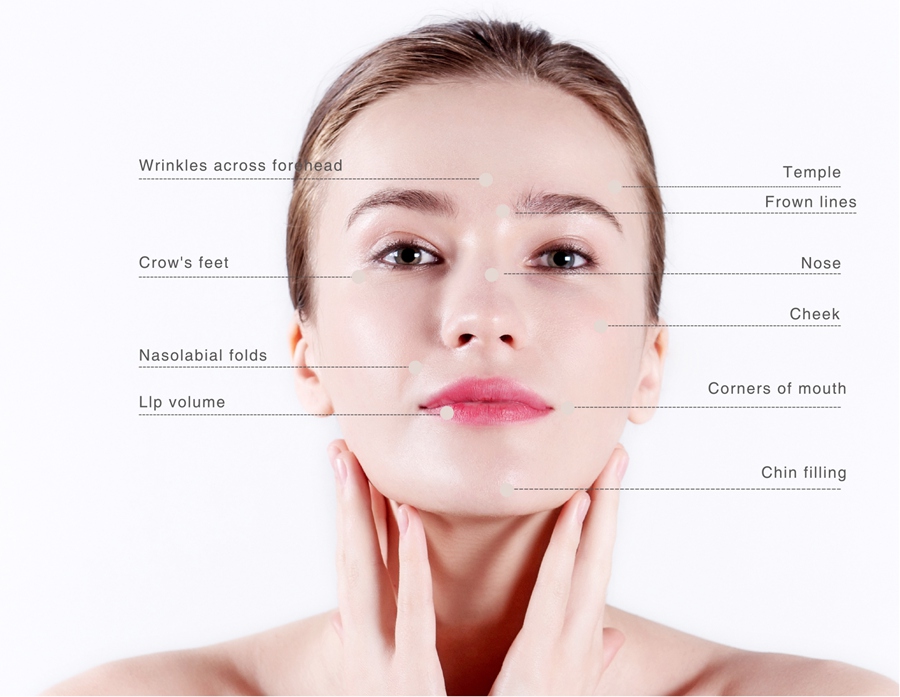
Facial fillers for sale offer a non-surgical way to rejuvenate your appearance by smoothing wrinkles and adding volume to your face.
Singderm® Mono-phasic Dermal Filler Composition
Composition (every 1ml)
Sodium hyaluronate 24mg
Lidocaine hydrochloride 3mg
Phosphate buffer pH7.2 q.s.1ml
Singderm® Mono-phasic Dermal Filler Advantages
Reliable HA raw material
Singclean's non-animal origin HA is JP (Japanese Pharmacopoeia) or EP(European Pharmacopoeia) approved.
Safety
With good biocompatibility, the osmotic pressure and PH value are close to the human body, minimizing redness and swelling.
Good Filling Effect
High viscoelasticity to achieve a good filling effect, not easily to shift and deform, offers strong supporting force to effectively improve uneven skin tone, enhance face shape and bring perfect and natural facelifts.
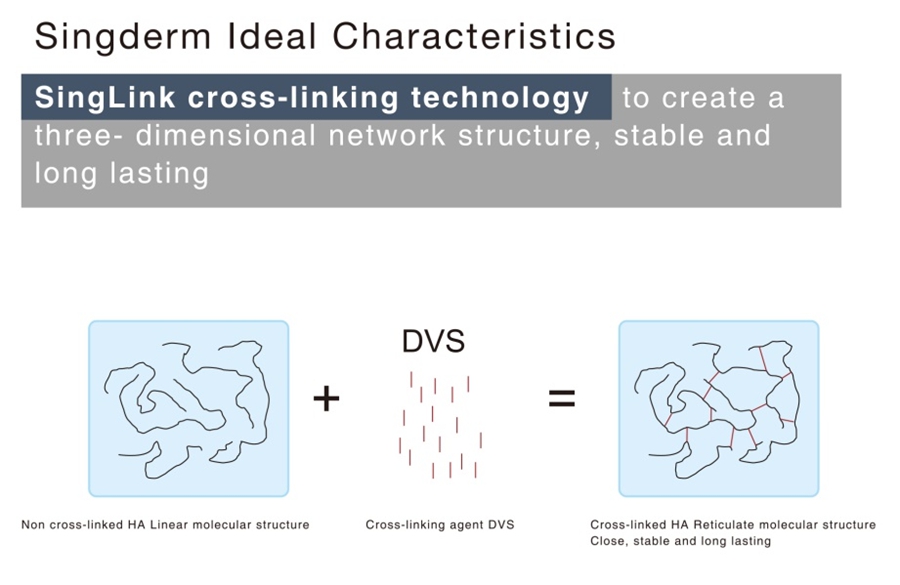
Long-lasting Effect
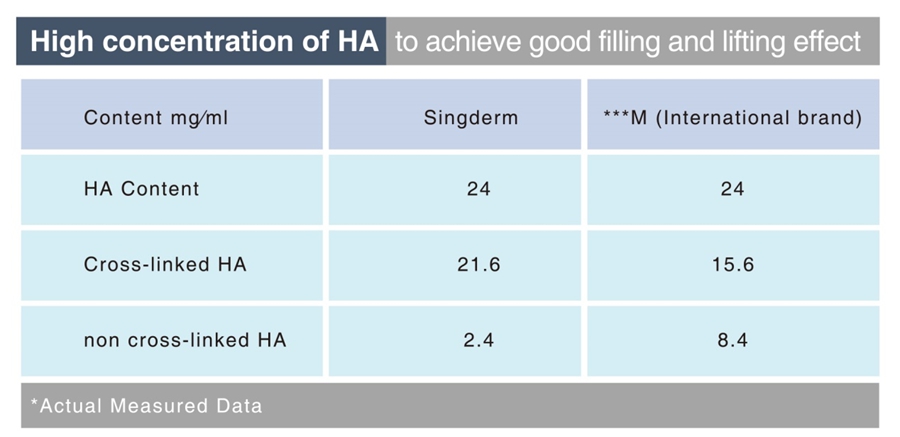
The high concentration HA of 24mg/ml and SingLink cross-linked technology guarantee a long-lasting effect that can last more than 12 months.
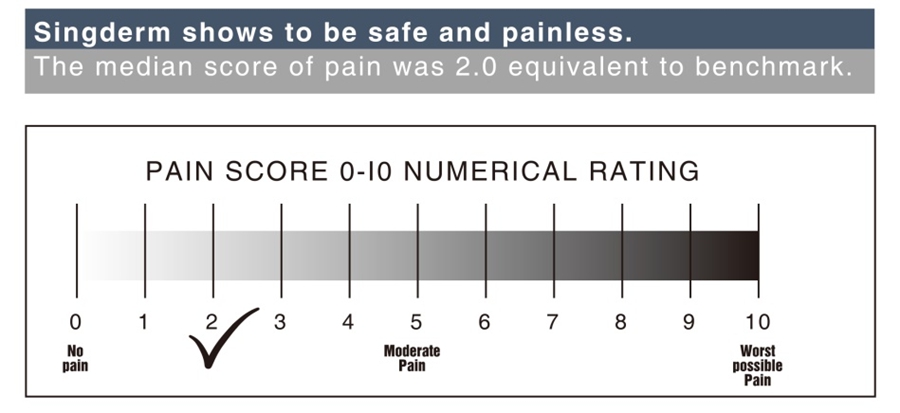
Pleasant and Painless Experience
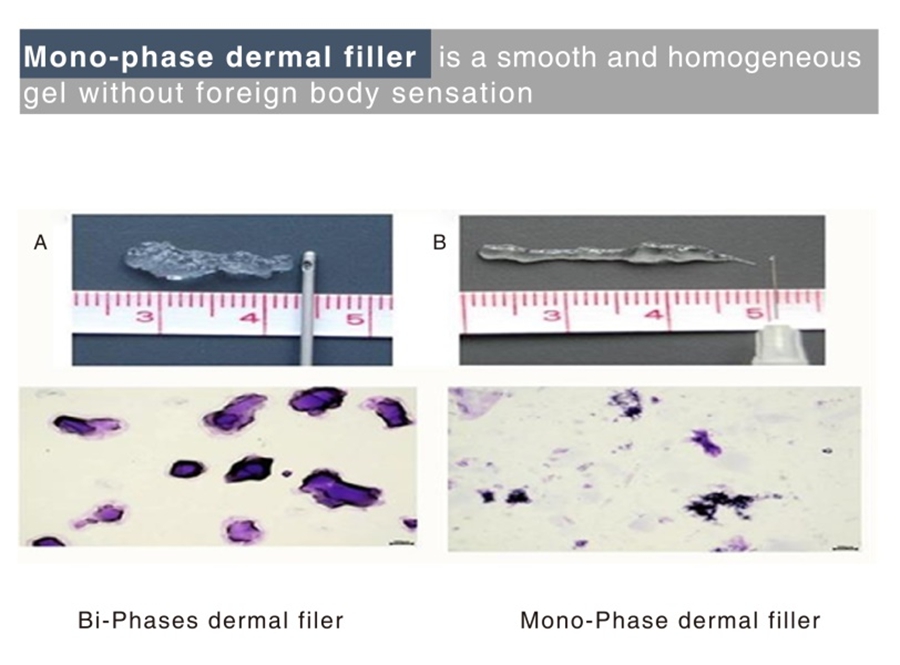
Singderm® Mono-phasic Dermal Filler is a mono-phasic, smooth and homogeneous gel, soft in texture without foreign body sensation.
0.3% lidocaine and evenly cross-linking giving the better experience of slight pain or even no pain.
You can find a variety of facial fillers for sale, including hyaluronic acid-based options, which are popular for their natural-looking results. For further information about our products and to locate a certified provider near you, please visit our official website or contact us today. Rediscover your radiance and experience the beauty of Singderm® Mono-phasic Dermal Filler.
Singderm® Mono-phasic Dermal Filler Treatment Effects
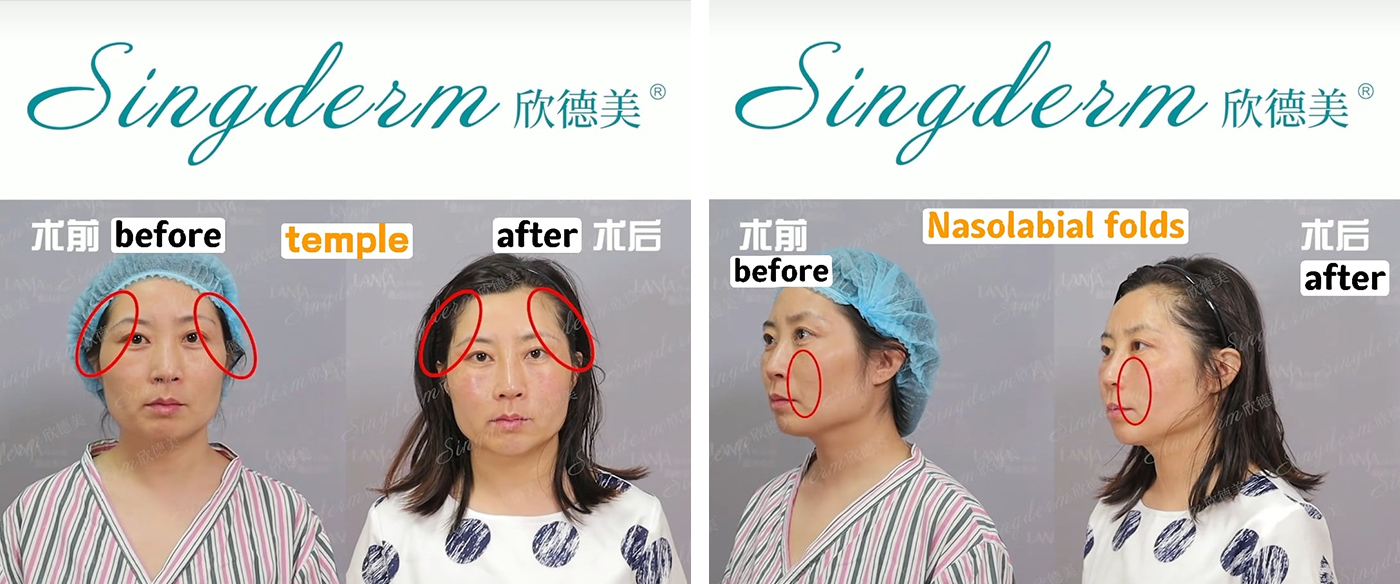

Singderm offers facial fillers for sale, ensuring safe and expert administration for a more youthful appearance.
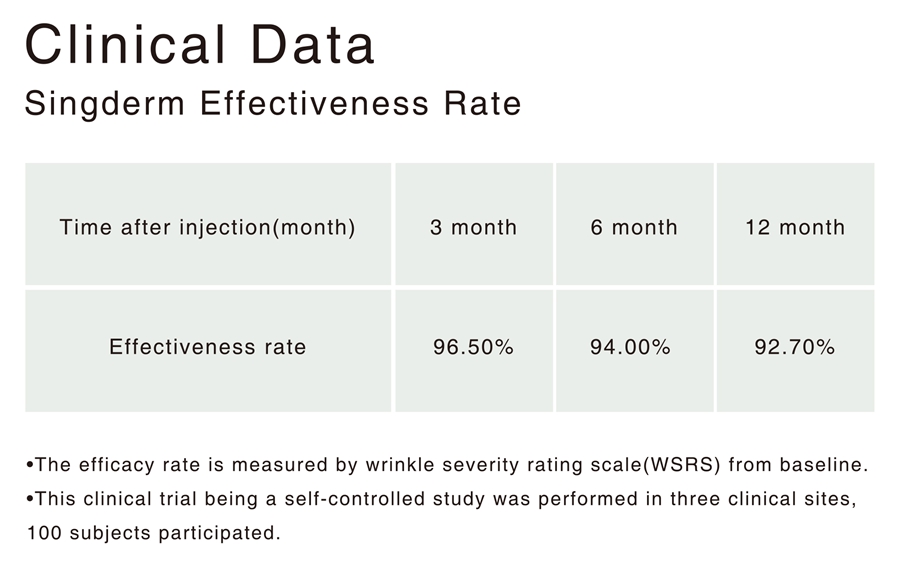
Singderm® Mono-phasic Dermal Filler Clinical Data
Competitive Advantage of Singderm
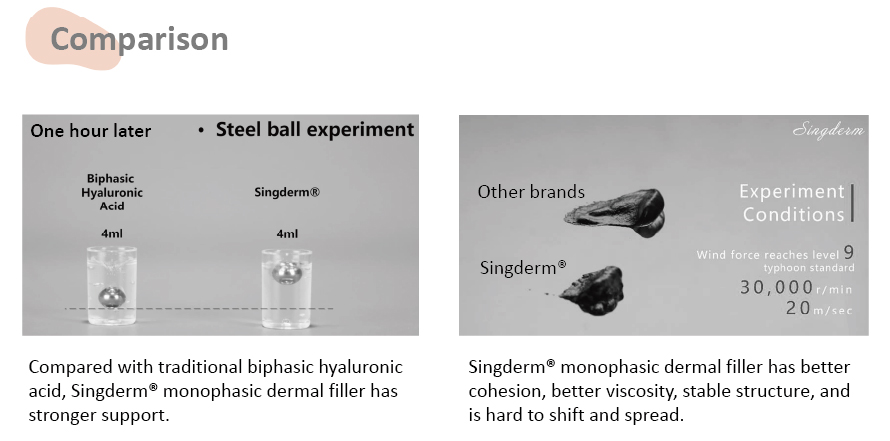
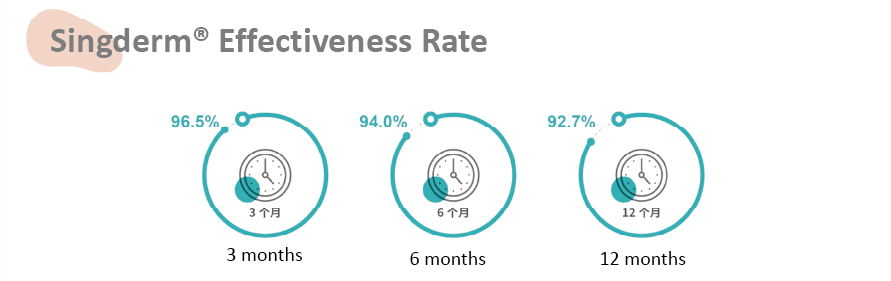
• The efficacy rate is measured by wrinkle severity rating scale(WSRS) from baseline.
• This clinical trial being a self-controlled study was performed in three clinical sites, 100 subjects participated.
Singderm® effectiveness rate is 92.7% at 12 month while currently the highest
12-month effectiveness rate of the internationally well-known dermal filler is 83%.
Singderm Facial fillers for sale can provide immediate rejuvenation, with minimal downtime and long-lasting effects.
Singderm® Mono-phasic Dermal Filler Specifications
Concentration: 24mg/ml

How to use Singderm® Mono-phasic Dermal Filler?
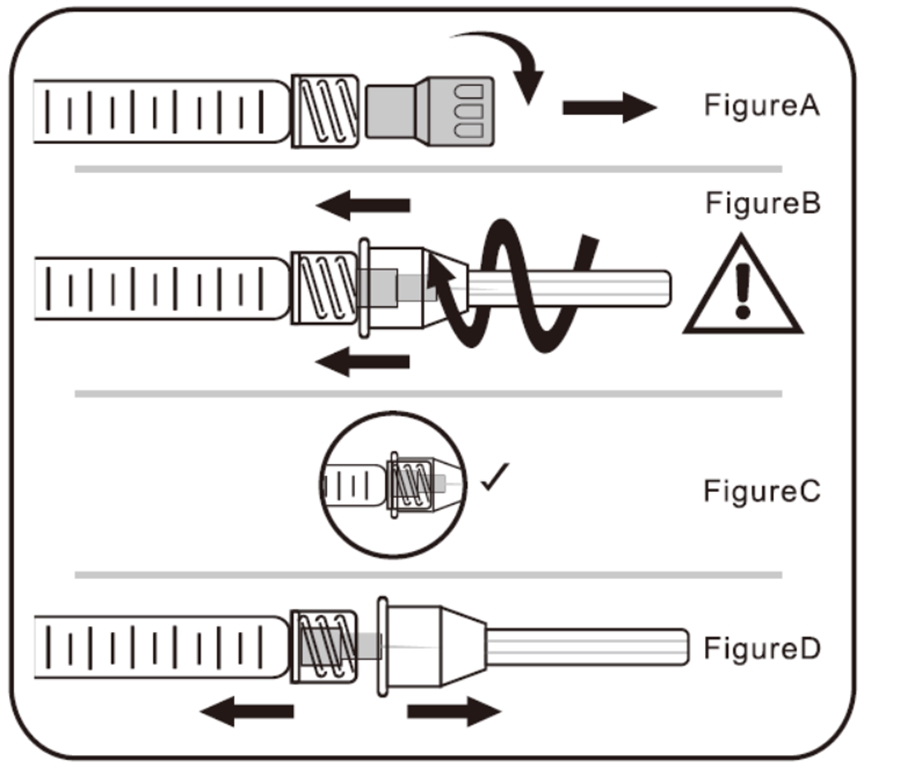
A. To Attach Needle to Syringe
STEP 1: Remove tip cap
Hold the syringe and pull the tip cap off the syringe as shown in Figure A
STEP 2: Insert the needle
Hold the syringe body and firmly insert the hub of the needle.
into the luer-lock end of the syringe.
STEP 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is seated in the proper position as shown in Figure C.
NOTE: Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needle cap as shown in Figure D.
B. Physician Instructions
1) This product is designed to be injected into the dermis or the mucous membrane of the lips by an authorized medical practitioner in accordance with local applicable regulations. As precision is essential to a successful treatment, the product must be used by medical practitioners who have undertaken specific training in injection techniques for filling.
2) Before starting treatment patients should be informed of the product's indications, contra-indications, incompatibilities and potential undesirable effects.
3) The area to be treated should be disinfected thoroughly prior to the injection.
4) Follow the above attaching needle to syringe steps, and depress the plunger rod until the product flows out of the needle.
5) After the first small amount of material has been injected into the patient, wait a full 3 seconds to allow the lidocaine to take effect before proceeding with the rest of the injection.
6) The injection technique may vary with regard to the angle and orientation of the bevel, the depth of injection, and the quantity administered. A linear threading technique, serial puncture injections, or a combination of the 2 have been used to achieve optimal results. Injecting the product too superficially may result in visible lumps and/or discoloration.
7) Inject Singderm® Mono-phasic Dermal Filler by applying even pressure on the plunger rod while slowly pulling the needle backward. The wrinkle should be lifted and eliminated by the end of the injection. It is important that the injection be stopped just before the needle is pulled out of the skin to prevent material from leaking out or ending up too superficially in the skin.
8) If the needle is blocked, do not increase the pressure on the plunger rod. Instead, stop the injection and replace the needle.
9) The amount injected will depend on the areas which are to be corrected. Correct to 100% of the desired volume effect. Do not overcorrect. The degree and duration of the correction depend on the character of the defect treated, the tissue stress at the implant site, the depth of the implant in the tissue, and the injection technique.
10) When injection is completed, the treated site should be gently massaged so that it conforms to the contour of the surrounding tissues.
11) With patients who have localized swelling, the degree of correction is sometimes difficult to judge at the time of treatment. In these cases, it is better to invite the patient to a touch-up session after 1 to 2 weeks.
12) Patients may have mild to moderate injection-site responses, which typically resolve in a few days. If the treated area is swollen immediately after the injection, an ice pack can be applied to the site for a short period.
13) After the initial treatment, an additional treatment (from 1 to 2 weeks later) may be necessary to achieve the desired level of correction. If the wrinkle needs further treatment, the same procedure should be repeated until a satisfactory result is obtained. The need for an additional treatment may vary from patient to patient and is dependent upon a variety of factors such as wrinkle severity, skin elasticity, and dermal thickness at the treatment site.
14) The physician should instruct the patient to promptly report to her/him any evidence of problems possibly associated with the use of Singderm®.
Storage Condition
Shelf life is 2 years, Store at 2°C to 30°C, DO NOT FREEZE.
Fragile.
Cautions for using of Singderm® Mono-phasic Dermal Filler
• Singderm® is packaged for single-patient use. Do not resterilize. Do not use if package is opened or damaged.
• Patients should be limited to 20 mL of Singderm® per 60 kg (130 lbs) body mass per year. The safety of injecting greater amounts has not been established.
• As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
• Singderm® is to be used as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, homogeneity, and performance of the product and it can therefore no longer be assured.
• The safety for use during pregnancy, in breastfeeding females, or in patients under 18 years has not been established.
• The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
• Singderm® should be used with caution in patients on immunosuppressive therapy.
• Patients who are using substances that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may, as with any injection, experience increased bruising or bleeding at injection sites.
• After use, treatment syringes and needles may be potential biohazards. Handle and dispose of these items in accordance with accepted medical practice and applicable local, state, and federal requirements.
• Singderm® injectable gel is a clear, colorless gel without particulates. In the event that the content of a syringe shows signs of separation and/or appears cloudy, do not use the syringe.
• If laser treatment, chemical peeling, or any other procedure based on active dermal response is considered after treatment with Singderm®, there is a possible risk of eliciting an inflammatory reaction at the indications site. An inflammatory reaction is also possible if the product is administered before the skin has healed completely after such a procedure.
• Failure to comply with the needle attachment instructions could result in needle disengagement and/or product leakage at the luer-lock and needle hub connection.
• If the needle is blocked, do not increase the pressure on the plunger rod but stop the injection and replace the needle.
• Athletes should be made aware that this product contains an active principle that may produce a positive result in anti-doping test.
• Medical practitioners must take into account the fact that this product contains lidocaine.
• The composition of this product is compatible with fields used for magnetic resonance imaging.
Singderm Feedback

Certification
NMPA, CE 2292, ISO 13485

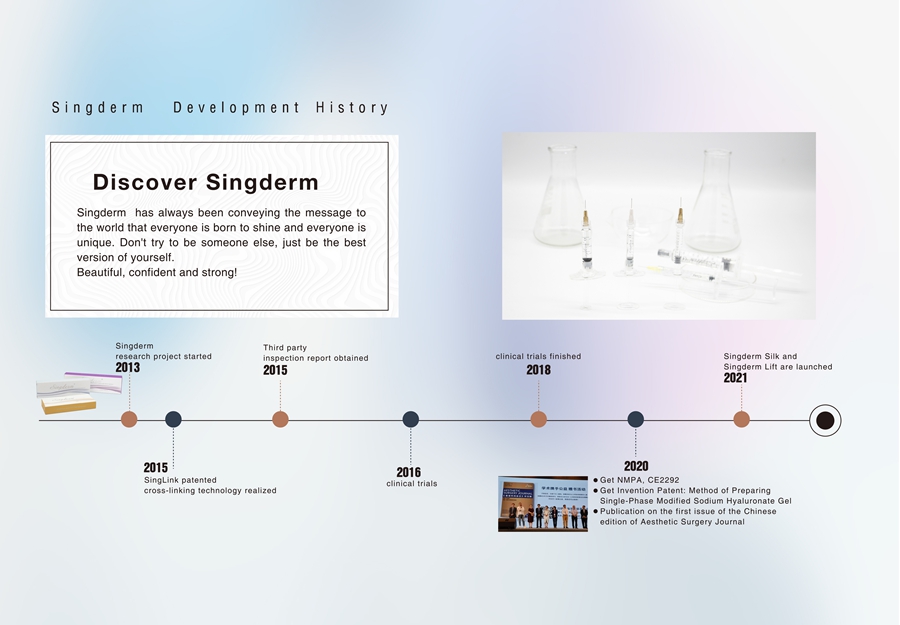
Singderm Development